i. : INTRODUCTION
Following the method outlined in "Journal of Chemical Education Vol. 71 pg 323-324, 1994" (including a number of changes for practical use), this investigation has set out to:
Create a practical method sheet (with results) which can be used by subsequent courses (e.g. BSc yr2 and Environmental Chemists).
The practicals involve reduction by Ascorbic acid, complexation with 1,5 Diphenyl carbazide and colorimetric techniques (spectrophotometry).
ii. : Table of contents
SECTION CONTENTS
i Introduction
ii Table of contents
1 CHROMIUM THEORY
1.i Why is chromium so important?
1.ii Complex theory
fig. 1 Complex form by model
fig. 2 Complex form by X-ray diffraction method
1.iii Final oxidation state after complexation
1.iv Methods of detection
1.v Toxicity of chromium (VI)
1.vi Complex colour: d - d shift or electron transfer
1.vii The original practical.
Re-print of Journal of Chemical Education, Vol.71 1994
1.viii Changes to the published method
Table 1 - Formulation of interfering ion solutions with Cr(VI)
2 SOLUTIONS
2.i Conversion of mg to ppm
2.ii H2SO4 0.18mol dm-3 & 3mol dm-3
2.iii 1,5 - Diphenyl carbazide.
2.iv l-Ascorbic acid
3 PRACTICAL
3.i Re-written practical
Table 2 - Chromium(VI)-DiPC and acid standards
Equation 1 - Conversion of absorbance from transmission
Table 3 - Formulation of interfering ion solutions with Cr(VI)
4 RESULTS
4.i The solutions
4.ii Calibration curve from standards
4.iii Water samples - collection points
4.iv Preparation of water samples
4.v Results from Cr(VI) solution used.
Table 4a - Calibration curve, week 1
Table 4b - Calibration curve, week 2
Table 4c - Calibration curve, week 3
4.vi Results from interfering ions
Table 5 - Interference from other ions
4.vii Results from water samples
Table 6 - Water sample absorbance values
4.viii Results from reduction by ascorbic acid
Table 7 - Reduction by ascorbic acid
4.ix Graphs
Graph 1 - Week 1 calibration curve
Graph 2 - Week 2 calibration curve
Graph 3 - Week 3 calibration curve
4.x Environmental question
5 CONCLUSION
APPENDICES
iii Bibliography
iv. Chromium data sheet
v. CHEMICAL DATA HAZARD SHEETS
vi. Graph from LJMU spectrophotometer & data settings.
vii. Thanks
viii. Technology used.
1. THEORY
1.i : Why is chromium so important?
Chromium metal is used in this country for a large variety of applications ranging from an additive in the manufacture of stainless steel to chromium plating (chrome plating) used for motorcycle exhausts and some older types of car bumpers to the colourisation of Rubies and Emeralds. Chromium metal has a more important use, it is a very hard transition metal and is normally amalgamated with titanium (another transition element) to make replacement hips in the USA and UK.
Chromium compounds (such as Chromic acid [a mixture of H2SO4 with sodium dichromate]) are used in the electroplating industry as both an additive and (in the case of chromic acid) as a highly powerful oxidising agent (chromic acid is roughly 3 times as powerful an oxidising agent as sulphuric acid due to the oxidising power of the Cr(VI) itself).
In the North West, there are only three chromium using plants. Two on Merseyside (both on the Wirral) and one in Manchester. As with any ore which comes in from other countries, the sightings of the plants reflect all the elements required for a successful plant; water (for raw materials processing and waste), a fine roads and rail infrastructure (transportation of other materials required as well as for sales of product) and cheaper power (Fiddler's Ferry and Salford Power generators are both near to the plants).
With all industrial processes, a waste product is inevitably formed. In the chromium industries (plating and manufacturing), it is normally the chromium (VI) compound (such as chromic acid and other high oxidising Cr(VI) cleaners). A smaller amount of the reduced Cr(III) and Cr(s) are released. The maximum permitted Cr(VI) in the UK is currently set at 50mg dm-3, with Cr(III) set at 1000mg dm-3. (1)
1.ii : Complex theory
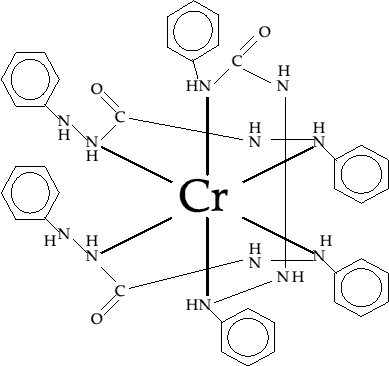
Cr(VI) is an element which will form octahedral complexes with ligands. In this experiment, the ligand is 1,5 diphenyl carbazide (DiPC). By constructing a molecular model of the ligand and positioning it around a suitable 6 branched element, the following complex is made (fig. 1). Notice that three of the DiPC ligands can be joined to the central Cr. This complex is extremely stable (see experimental details for further information). A second structure has also been proposed(2) (shown in fig. 2) with the Cr being 'sandwiched' between the delocalised rings on the primary benzene rings. The apparent conflicts between the two theories for the structure are due to one version being borne out of models (as in fig. 1) while the second is as a result of the use of x-ray diffraction techniques which gave the second result.

fig.1. Complexation model
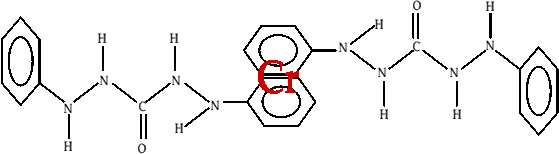
fig. 2 X-Ray diffraction of the DiPC-Cr(VI) complex
The colour of the compound is as a result of electron transfer, rather than d orbital shifts (see Complex colour - d-d shift or electron transfer? for explanation.)
1.iii : Final oxidation state of the complex
As described, the DiPC will not complex with the Cr(III) to form a colour. The reason is due to the stability of the Cr(III) ion and it's subsequent chemical inertness (as described previously). This therefore means that the final oxidation state of the Cr complex must be Cr(VI)(3).
1.iv : Chromium detection
In it's most stable form (Cr (III)), chromium can be detected by AA, by gravimetric analysis with a number of substances (such as hydrolysis of potassium cyanate to form the insoluble hydroxide(4)).
Cr (VI) can also be detected by AA and also by titration with standard Na2S2O4 with I2(4).
If the solutions to be tested were concentrated enough (above 0.01M), then analysis by titration or gravimetric techniques could be considered. AA cannot be considered as this will only determine Cr (any oxidation state).
In water samples, the maximum permitted level in this country is 50mg dm-3 for Cr(VI). As this is far below 0.01M, only one of two methods can be considered.
The first is rather impracticable. X-Ray Crystal photography. This would be very expensive and very long to perform.
A quicker and easier method will be the complexation of Cr(VI) with 1,5 - Diphenyl carbazide and the determination of concentration colorimetrically. The carbazide will form a very strongly coloured compound.
Cr(VI) will absorb best at 540nm(5). The absorbtivity with the complex is 40000 dm3 g-1 cm-1 at 540nm(5). Even without a colorimeter, the complex is strongly enough coloured for a relatively able person to compare one concentration to another.
1.v : Reasons for toxicity of Cr (VI) compared to Cr (III)
Cr (III) is a very stable oxidation state for chromium. In this state, the chrome is labile and kinetically very slow to react or form complexes. It is not a strong oxidiser and the human's natural body acidity is enough for the chrome to keep to this Cr (III) state.
Cr (VI) is a different story.
Cr (VI) is not a very stable state when compared to Cr(III). The Cr (VI) is a very strong oxidising agent (therefore very fast in reacting, unlike Cr (III) and likely to form complexes). This is not why Cr (VI) is toxic.
One of the reduction products of Cr (VI) is Cr (V). Chrome (V) is a known carcinogen(6) and will lodge in any tissue to form cancerous growths. There are reports that chromium (V) is also a factor leading to premature senility in parts of Russia(7). This has not been substantiated by the UN or any other academic group.
In the body, the acidity and action of enzymes on Cr (VI) will promote the formation in small quantities of Cr (V)(8). However, as the size of this is normally too large to be adopted by a tissue, the Cr (V) will pass out. The only place where the Cr (V) is likely to lodge is in some of the fine capillaries in either the kidneys, intestines or lungs.
During the passage out, Cr (VI) will continue to oxidise anything it can, leaving deposits of the relatively safe Cr (III) and completely unsafe Cr (V) behind.
Even at the concentrations used for this experiment, the levels of Cr(VI) will pose a health risk and so all protective methods MUST be employed (see hazard sheets for details).
1.vi : The complex colour - d-d shift or electron transfer?
By calculating the molar absorption, A, it is possible to calculate if it is a d-d shift. However, it should be noted that Cr has the configuration of 3d5 4s1 and so Cr(VI) will have the d0 configuration. Without anything in the d orbital to shift, the colour has to be due to electron transfer, therefore, the following may seem to be a bit redundant!.
The complex contains 0.00127g of Cr(VI). Convert this into molarity by division with the RMM of Cr.
Ans : 2.4 x 10-5 M. This is c.
We know the path length is 1 and that e = 40000 dm3 g-1 cm-1 (5)
Feeding this into the formula : A = ecl, A turns out to be 0.48.
With e being 40000 (4 x 104) and this value of A = 0.48, combined with the maximum absorption of the complex being just below 1, all of the indicators point to charge transfer giving rise to the intense colour of the complex.
1.vii : The original practical.
This has been reproduced from The Journal of Chemical Education, Volume 71, Number 4, April 1994 pgs 323-324. The text has only been changed to be in English (rather than American English) and for the inclusion of S.I. units. All other text remains the same. The superscript numbers are for the text and not for the main body of this project.
FILTRATES AND RESIDUES
TESTING THE WATERS FOR CHROMIUM
MARY S. HERRMANN, University of Cincinnati - Raymond Walters College.
Frequently, metal ions are introduced into waterways by industry as waste from various processes. Many of the metal ions are toxic to humans, and their release must be monitored and controlled carefully.
A metal ion that can be a pollutant is the hexavalent chromium ion. There are two natural forms of ionic chromium, the hexavalent ion, Cr(VI) and the trivalent Cr(III). Cr(III) is much less toxic than Cr(VI) and seldom found in potable waters. Cr(VI), however, is toxic to humans and is found in water. It has been shown to toxic when in aerosol form causing damage to the skin and upper respiratory system and causing lung cancer1. The toxic effects from Cr(VI) in drinking water are not well documented, but it is a suspected carcinogen.
There are many industries that use chromic acid and other forms of Cr(VI) and are possible sources of Cr(VI) pollution in either water or air or both. One industry that pollutes water with Cr(VI) is the chrome-plating industry (for the plating of car bumpers). Chromic acid is used in the electroplating process and can be present in industrial waste waters. Cr(VI) also can enter water supplies from industrial cooling towers where chromic acid is added to the water to inhibit metal corrosion. The Environmental Protection Agency recently banned Cr(VI) from use in 37,500 building roof cooling towers (that leak coolant into the air) in the United States that had caused an estimated 20 cancer deaths2. Some other products that contain Cr(VI) are paints, pigments, tanning agents, inks, fungicides and wood preservatives3.
The maximum permissible level or Cr(VI) allowed to be released into the waterways is 50mg dm-3. It level in drinking water normally is much lower and a lever higher than 3mg dm-3 is suggestive of industrial pollution.
The experiment outlined here is a test for the presence of Cr(VI) in water that uses a sensitive colorimetric reagent. Students determine the level of Cr(VI) in both the local tap water and some polluted "industrial" waste water. The experiment also investigates some methods by which industry can lower Cr(VI) concentrations prior to releasing their waste water.
Materials
Chromium (VI) solution (1.27mg dm-3 Cr(VI)
To prepare place 3.6mg of K2Cr2O7 and 10cm3 of conc. sulphuric acid into about 500cm3 distilled water in a volumetric flask. Dissolve and then add distilled water to a final volume of 1dm3.
Polluted water (dilute 100cm3 Cr(VI) solution to 1dm3 with distilled water)
Diphenyl carbazide solution (0.50g in 200cm3 propanone)
Ascorbic acid solution (0.2g in 100cm3 distilled water)
0.18mol dm-3 sulphuric acid solution
To prepare, add 10cm3 of conc. sulphuric acid to about 500cm3 with distilled water in a volumetric flask. Mix and add distilled water to a final volume of 1dm3.
3.0mol dm-3 sulphuric acid solution
Add 42cm3 conc. sulphuric acid to about 150cm3 of distilled water in a 250cm3 volumetric flask. Mix and add distilled water to make a final volume of 250cm3.
Pipette, 0.5cm3
Graduated cylinder, 10cm3
Visible spectrophotometer and cells, if available.
Student safety and disposal
GOGGLES MUST BE WORN THROUGHOUT THE EXPERIMENT. Although low concentrations and small volumes are used, all disposal must be disposed of by local guidelines.
Procedure
Preparation of standards
1. Obtain six test tubes capable of holding 15-20cm3 and label them 0, 1, 2, 3, 4 and 5. Add to these test tubes the quantities of Cr(VI) and the 0.18mol dm-3 sulphuric acid according to the table below using separate 10cm3 graduated cylinders. Stopper and mix the contents of each test tube by shaking.
| Tube number | 0 | 1 | 2 | 3 | 4 | 5 |
| CrVI cm3 | 0.0 | 0.4 | 1.0 | 2.0 | 4.0 | 10.0 |
| H2SO4, 0.18M cm3 | 10.0 | 9.6 | 9.0 | 8.0 | 6.0 | 0.0 |
2. To each test tube, pipette 0.5cm3 of diphenyl carbazide solution. Mix the contents of the test tubes, and let them stand for five minutes for colour development.
3. If a spectrophotometer is available, measure the absorbtivity of each sample at 540nm, and plot a standard curve. For the blank, use tube 0. The absorbtivity for the diphenyl carbazide-Cr(VI) solution is 40,000 dm3 g-1 cm-1 at 540nm4. If no spectrophotometer is available, save the standard solutions for colour comparison in the determination of chromium in water samples.
Determination of Chromium in water samples
1. For each sample to be tested, obtain a test tube and label it. Place 10cm3 of the water sample in the test tube. The "polluted water" should be tested as well as any other samples available.
2. To each test tube, add 12 drops of 3M sulphuric acid.
3. To each tube, pipette 0.5cm3 of diphenyl carbazide solution and allow 5 minutes for colour development.
4. Determine the amount of Cr(VI) present either by absorbance at 540nm or by visual comparison with standard solutions.
Reducing Chromium(VI) levels for disposal.
Industries use a variety of methods to reduce the Cr(VI) concentration to levels permissible for disposal. This section describes two methods for reducing the concentration of the polluted water. Students may wish to try other methods as well.
Dilution method
The maximum permissible level of Cr(VI) allowed to be released is 50mg dm-3. Assume an industry has 100dm3 of Cr(VI) polluted water at the same concentration as the polluted water from the determination of chromium in water samples. Calculate how many litres of chromium free water must be mixed with the polluted water so that it can be released (ans - add around 150dm3 of Cr-free water.)
Reduction Method
Cr(VI) is reduced easily to Cr(III) that can be released at the much higher level of 1000mg dm-3. Take a sample of polluted water and add 5 drops of ascorbic acid solution (a mild reducing agent). Swirl to mix and determine the Cr(VI) concentration as you did in the part above. Many other methods of reduction are possible5.
Variation to experiment
A variation in the above procedure that teachers may choose to use involves a bit more preparation time but will be more meaningful to students. The variation presents students with a Cr(VI) pollution mystery that they are to solve. Students are given a map prior to performing the experiment and told that at location seven on the map an unusually high level of Cr(VI) was discovered in the river water (200mg dm-3). The map in of a hypothetical town, Anytown, and some surrounding industries. The students will be testing Cr(VI) levels in the river water at the various sites indicated in order to locate sources of the pollution.
Materials for variation
The above materials will be used except that the solutions will be substituted for the polluted water.
Label six jars (mayonnaise jars or similar) with the numbers 1 through to 6. Place the following solutions into the appropriate jar.
1 and 2 : 500cm3 of unpolluted water (distilled water or tap water known to be free of Cr(VI))
3 : 250cm3 Cr(VI) solution and 250cm3 unpolluted water.
4 : 150cm3 Cr(VI) solution and 350cm3 unpolluted water.
5 and 6 : 100cm3 Cr(VI) solution and 400cm3 unpolluted water.
Procedure for variation
The procedure is identical to above except that solutions 1-6 are substituted for "polluted water" in the determination of chromium water samples in the first part of the experiment.
Literature Cited
1 Varma, M. M.; Serdahely, S.G.; Katz H.M. J. Envir Health 1976, 39 (Sept/Oct.). pp 90-100
2 Cooper, M. NCI Cancer Weekly, Jan. 15, 1990, p.12
3 Chromium: National Academy of Sciences, Washington DC, 1974
4 Standard methods for the examination of water and wastewater: 17th ed. American Public Health Assoc., Washington DC, 1989
5 Lunn, G.; Sansone, E.B. J. Chem. Educ. 1989 66, 443
1.viii : Changes to published practical
Due to the fact that the experiment outlined is a very much 'bare bones' experiment, a number of changes are required.
CHANGES TO MATERIALS REQUIRED.
3.6mg of K2Cr2O7 is a minute amount (0.0036g) and would be very difficult to accurately weigh (even with the best will in the world!!.). However, it would be inpractible to multiply the quantities of all reagents by 100 to achieve 0.36g of solid, 1dm3 conc. sulphuric acid to a final volume of 10dm3.
A better solution to this problem would be to use a serial dilution method. This may introduce errors, but at HC2/BSc 2 level, these should be negligible.
| Compound | RMM | 1M soln | 1ppm | 0.1ppm | 0.01ppm | 1.27ppm | Total |
| Cobalt Sulphate | 281.0972 | (g) | (g) | (g) | (g) | (g dm-3) | |
| Co | 58.9332 | 58.9332 | 0.001697 | 0.000170 | 0.000017 | 0.002115 | |
| SO4 | 96.0576 | 96.0576 | 0.001041 | 0.000104 | 0.000010 | 0.001322 | |
| 7 Water | 126.1064 | 126.1064 | 0.000793 | 0.000079 | 0.000008 | 0.001007 | 0.004484 |
| Mercury Chloride | 271.4960 | ||||||
| Hg | 200.59 | 200.59 | 0.000499 | 0.000050 | 0.000005 | 0.000633 | |
| Cl2 | 70.906 | 70.906 | 0.001410 | 0.000141 | 0.000014 | 0.001791 | 0.002424 |
| Barium Chloride | 294.1844 | ||||||
| Ba | 137.33 | 137.33 | 0.000728 | 0.000073 | 0.000007 | 0.000925 | |
| Cl2 | 70.906 | 70.906 | 0.001410 | 0.000141 | 0.000014 | 0.001791 | 0.002716 |
| Potassium Dichromate | 294.1844 | ||||||
| K | 78.1966 | 78.1966 | 0.001279 | 0.000128 | 0.000013 | 0.001624 | |
| Cr | 103.992 | 103.992 | 0.000962 | 0.000096 | 0.000010 | 0.001221 | |
| O | 111.9958 | 111.9958 | 0.000893 | 0.000089 | 0.000009 | 0.001134 | 0.003979 |
| Iron (III) Chloride | 270.2972 | ||||||
| Fe | 55.847 | 55.847 | 0.001791 | 0.000179 | 0.000018 | 0.002274 | |
| Cl3 | 106.359 | 106.359 | 0.000940 | 0.000094 | 0.000009 | 0.001194 | |
| 6 Water | 108.0912 | 108.0912 | 0.000925 | 0.000093 | 0.000009 | 0.001175 | 0.004643 |
| Amm. Iron (II) Chloride | 392.130 | ||||||
| Fe | 55.847 | 55.847 | 0.001791 | 0.000179 | 0.000018 | 0.002274 | |
| Amm. Sulphate | 228.1918 | 228.1918 | 0.000438 | 0.000044 | 0.000004 | 0.000557 | |
| 6 Water | 108.0912 | 108.0912 | 0.000925 | 0.000093 | 0.000009 | 0.001175 | 0.004006 |
The required Cr(VI) in acid concentration is 1.27mg dm-3 (or 1.27 ppm). The sulphuric acid has only been added to stabilise the Cr in the +6 oxidation state. Therefore, if the method was followed but with 0.360g of Cr(VI) used and all the rest added when this had been diluted down by a factor of 100 (10cm3 of the Cr(VI) solution to a 1dm3 flask will result in 3.6mg), the required solution can be made with a great deal more accuracy and ease.
A Cr(III) solution is also required as well as a mixed metal ion solution to test if these will interfere with the actual determination of Cr(VI). These can be made up as above with the following chemicals. If the concentrations are all kept the same, then the testing can be a fairer tests (will equal concns of Mx+ interfere with the Cr(VI)?).
CHEMICALS : Mercury (II) Chloride (S1 poison - care!), Iron (II) Chloride, Iron (III) Chloride, Barium Chloride, Cobalt (II) Sulphate, Potassium Chromate.
These have been chosen as they are the most likely ions to be found in British Waterways. They are also coloured (except for barium and mercury salts).
PROCEDURAL CHANGES.
Preparation of standards.
This will remain unchanged for the Cr(VI) curve.
To determine if the hypothesis of metal ion interference is valid or not, the following tests should be performed.
A further 9 tubes are set up as in the table below. The procedure is then the same as before.
| Tube No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| CrVI | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| H2SO4 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Mx+ | 0.0 | 5.0 Hg | 5.0 FeII | 5.0 FeIII | 5.0 Ba | 5.0 Co | 2.5 FeII | 2.5 Hg | 2.5 Co |
| Mx+ | 2.5 FeIII | 2.5 CrIII | 2.5 Ba |
Table 1. Formulation of interfering ion solutions with chromium (VI)
Determination of Chromium in water samples
To 2. in the published method, a volume should be inserted to replace the phrase "a drop". This will be x cm3 where x is the correct amount. A drop is a rather haphazard method of addition as a drop can vary greatly from the method of dropping (a Pasteur pipette will dispense 0.1cm3 per drop, while a plastic disposable will dispense up to 0.19cm3 with every possible inbetween!.)
As most of the water samples are from natural sources (rather than, say, out of a tap), it may be necessary to prepare the water. This can be done quickly by performing the following:
(1) Take 50cm3 of the water sample and filter under pressure using a Buchner set up (this is more for speed than anything else!).
(2) To remove any waste organic materials and to acidify the water, add 10cm3 of conc. sulphuric acid. All organic solids and miscible liquids will now have been oxidised. Any alkalinity (which will favour the Cr(III) state more hence the addition of the 3mol dm-3 to all of the water samples to ensure the Cr(VI) state) will have been removed.
(3) Re-filter under pressure to remove any carbonised/oxidised material.
The chromium sample is now ready for complexation with the DiPC. Further addition of the 3M sulphuric acid is not needed as the solution will now be sufficiently acidic to keep the Cr(VI) state.
2 : SOLUTIONS
2.i : Conversion of mg to ppm
By taking the RMM of the components of the chemical, it is easy to calculate how much of each will be in a 1M solution. A 1ppm solution of any single element will contain 0.001g of it per dm3, the same can be said for a 0.1 and 0.01ppm solutions. By finding these out for all of the components, adding the results together (1*1ppm + 2*0.1ppm + 7*0.01ppm for each), the mass of the solid required for the 1.27ppm solution is obtained.
Below is for all of the compounds used in my experiments. Note the K2Cr2O7 is not the same value as for the printed value. This is due to errors in values stated by the chemical companies. I have calculated them using the values given by IUPAC in 1969 (based on 12C).
| Potassium Chromate | 139.0925 | 1.27ppm | Total | ||||
| Symbol | RMM | 1M | 1ppm | 0.1ppm | 0.01ppm | (g) | (g dm-3) |
| K | 39.0983 | 39.0983 | 0.003248 | ||||
| Cr | 51.996 | 51.996 | 0.002442 | ||||
| O3 | 47.9982 | 47.9982 | 0.002650 | 0.008340 |
2.ii : Sulphuric acid, 0.18 and 3 mol dm-3
For both of these, the concentrated acid is used first. This must be handled with great care. All volumes of this must be pipetted out in perfectly dry pipettes.
0.18 mol dm-3 : Use 10cm3 acid, dilute to a final volume of 1dm3.
3.0 mol dm-3 : Use 42cm3 acid, dilute to a final volume of 250cm3.
2.iii : 1,5 Diphenyl carbazide (DiPC)
The method says to use 0.5g diluted to 200cm3 in propanone. By calculating how much DiPC is in each 50cm3, an easier value (for dilution) of 0.625g diluted to 250cm3 is obtained.
This solution must be made in a fume cupboard due to the solvent being propanone. It must also be stored away from light.
2.iv : l-Ascorbic acid
0.2g was diluted in 100cm3 water.
3 : Method.
ANALYSIS OF CHROMIUM IN WATER9
INTRODUCTION
Chromium (VI) is a toxic pollutant in our waterways. The experiments that follow are to be carried out and from the results, the effectiveness of the method can be determined and the suitability of the test.
CHROMIUM DETECTION
For the purpose of the major part of the first part of the experiments, the method of detection is by colorimetric techniques. The chromium is first to be complexed with a solution of 1,5 diphenyl carbazide (DiPC) in propanone, acidified and then, tested in a spectrophotometer set at 540nm.
The DiPC will not complex with Cr(III). This test is therefore specific to Cr(VI).
Q. Why is it specific to Cr(VI) and not Cr(III)?
A. Cr (III) is a very stable oxidation state for chromium. In this state, the chrome is labile and kinetically very slow to react or form complexes. It is not a strong oxidising agent. Cr (VI) is a different story. To begin with, Cr (VI) is not a very stable state when compared to (III). The Cr (VI) is a very strong oxidising agent (therefore very fast in reacting, unlike Cr (III) and likely to form complexes).
PREPARATION OF THE Cr(VI) STANDARD SOLUTION (1.27mg dm-3)
NOTE : Cr(VI) AND IT'S REDUCTION PRODUCT (Cr(V)) ARE BOTH VERY HAZARDOUS TO HEALTH. THEY HAVE BEEN SHOWN TO BE CARCINOGENIC BY INHALATION, SKIN ABSORPTION AND IF TAKEN ORALLY. ALL SAFETY MEASURES MUST BE OBSERVED (GLOVES, EYE PROTECTION AND LAB COATS MUST BE WORN.)
1. Carefully weigh out 0.36g of AR potassium dichromate into a 100cm3 beaker. Dissolve this in about 75cm3 of deionised water. Transfer this to 1dm3 volumetric flask, washing the beaker as many times as required to ensure that all of the dichromate has been transferred to the flask. The stirrer used, must also be rinsed into the flask.
2. Fill the flask to just beneath the graduation mark with deionised water. Stopper the flask and invert six or seven times. Unstopper the flask and leave to stand for a couple of minutes. Fill to the graduation mark, stopper, invert six or seven times. Let the solution settle.
3. Pipette a 10cm3 portion into a clean, fresh 1dm3 flask, followed by 10cm3 of 0.18mol dm-3 sulphuric acid. Repeat instruction 2 and label as "Cr(VI) standard solution"
The sulphuric acid solutions (0.18mol dm-3 and 3.0mol dm-3) are supplied.
DiPC has to be made up freshly and stored in an amber bottle as it is very light sensitive. To prepare the stock solution, accurately weigh 0.625g of the solid and dissolve in the minimum amount of propanone. Transfer the solution to a 250cm3 volumetric flask and dilute to the mark with propanone. The solution must be kept stoppered when not in use. Propanone will readily evaporate and therefore the concentration of the solution will alter.
Construction of a calibration curve with a spectrophotometer.
1. Obtain six test tubes and label 0 to 5. Pipette into each tube the quantities of solutions outlined below. Mix the solutions well. DO NOT COVER THE MOUTH OF THE TUBE WITH THE FINGER. (This will make the concentration of each tube different to the experiment and therefore effect the end result. Cr(VI) is also a known carcinogen).
| Tube number | 0 | 1 | 2 | 3 | 4 | 5 |
| CrVI cm3 | 0.0 | 0.4 | 1.0 | 2.0 | 4.0 | 10.0 |
| H2SO4 0.18M cm3 | 10.0 | 9.6 | 9.0 | 8.0 | 6.0 | 0.0 |
Table 2. Cr(VI)-DiPC & acid standards.
2. To each tube, pipette 0.5cm3 of DiPC solution. Mix the contents and let them stand out of direct light for 5 minutes to develop. It is vital that they are left for 5 minutes as the solutions will continue to complex for this length of time (ie maximum complexation takes place by 5 minutes).
3. With the spectrophotometer set at 540nm, fill a cuvette with each solution and measure the absorbtivity. The blank should be from tube 0. The absorbtivity for the complex is 40000 dm3 g-1 cm-1. On the machine, the control should be turned to "Absorbance" or "A". If it only has Transmission, the following formula should be employed to calculate the absorbance:
![]()
Equation 1 - Conversion of absorbance from transmission.
4. From the above results, plot a graph of absorption against concentration. This is the calibration curve for the complex and should be made before carrying on with the experiment.
THE EFFECT OF INTERFERING METAL IONS
Water will not solely contain Cr(VI). It is known to contain (amongst other things) such as lead (II), chromium (III), cobalt, iron (II), iron (III), mercury and barium. The second set of experiment is to determine if these will have any effect on the absorbtion. As in all cases, the elements should only be in trace amounts, for the purposes of this test, the concentrations will be same as for the chromium (VI).
PREPARING THE SOLUTIONS
Following the method outlined for the Cr(VI) solution above, weigh out the masses of the following salts :
CoSO4 (0.2242g), HgCl2 (0.1212g) (WARNING : this is a schedule 1 poison. Great care must be employed when handling), Ammonium iron(II) sulphate (0.2003g), Iron (III) chloride (0.2321g), BaCl2 (0.2358g) and KCrO3 (0.4170g).
Accurately make these up to a final volume of 500cm3. Prepare the final solution as before except only use 5cm3 instead of 10cm3 for each solution.
DETERMINATION OF ION INTERFERENCE.
This will require another 9 tubes set up as follows:
| Tube No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| CrVI | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| H2SO4 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Mx+ | 0.0 | 5.0 Hg | 5.0 FeII | 5.0 FeIII | 5.0 Ba | 5.0 Co | 2.5 FeII | 2.5 Hg | 2.5 Co |
| Mx+ | 2.5 FeIII | 2.5 CrIII | 2.5 Ba |
Table 3 - Formulation of interfering ion solutions with Cr(VI)
To each of the tubes, add 0.5cm3 of the DiPC solution. Leave for five minutes and then pour into a cuvette. Measure the absorbtion at 540nm.
DETERMINATION OF CHROMIUM IN WATER SAMPLES
You have been given a set of water samples to test for the presence of Cr(VI). This can be carried out essentially as for the calibration curve but with a number of differences:
1. 0.5cm3 of 3.0mol dm-3 H2SO4 must be added to the water sample.
2. One of the water samples given is very murky. This is not unusual as most streams and canals will have bits of plants and also micro-organisms in it. To eliminate these, perform the following:
(1) Take 50cm3 of the water sample and filter under pressure using a Buchner set up
(2) To remove any waste organic materials and to acidify the water, add 10cm3 of conc. sulphuric acid. All organic solids and organic miscible liquids will now have been oxidised.
(3) Re-filter under pressure to remove any carbonised material.
The experiment can now proceed. Measure the absorbance of the test solution and from comparison of the calibration curve, calculate the concentration of each water sample.
REDUCTION OF CHROMIUM LEVELS
As previously stated, Cr(VI) will have an adverse environmental impact on marine life as well as our own. The following 3 experiments will show how to reduce the levels.
REDUCTION METHOD 1. DILUTION METHOD
The maximum permissible level of Cr(VI) allowed to be released is 50mg dm-3. Assume an industry has 100dm3 of Cr(VI) polluted water at the same concentration as the polluted water from the determination of chromium in water samples. Calculate how many litres of chromium free water must be mixed with the polluted water so that it can be released.
REDUCTION METHOD 2. CHEMICAL REDUCTION METHOD 1.
As stated above, the maximum permissible level of Cr(VI) allowed to be released is only 50mg dm-3. However, by reacting with 0.2cm3 of l-ascorbic acid (0.2g in 100cm3 of distilled water) BEFORE complexation, the Cr(VI) will be reduced to Cr(III) which can be released at the far higher level of 1000mg dm-3.
To verify this, take tube 5 from the calibration curve experiment. From this, pipette of 3cm3 and add the ascorbic acid and mix. Pipette in 0.5cm3 DiPC and leave for five minutes.
While this is developing, take a second 3cm3 from the same tube, add the DiPC and mix. Now add the ascorbic acid and leave to develop.
To verify, record the observed absorbtion of both and compare from the calibration curve data.
REDUCTION METHOD 3. CHEMICAL REDUCTION METHOD 2.(10)
Take a 50cm3 aliquot of the Cr(VI) solution and add to this 10cm3 of 10% sodium metabisulphite (Na2S2O5). Neutralise with magnesium hydroxide solid until all of the effervescing ceases.
Take a 5cm3 aliquot of the neutralised solution and transfer to a clean test tube. Add 0.5cm3 of DiPC and test at 540nm for an absorbtion peak. No addition of the 3M sulphuric acid is needed.
CALCULATION OF RESULTS
The following are required from you for the results :
i. A calibration curve of [Cr(VI)] v absorbtion.
ii. Using the linear regression program (least mean squares), find the error in the calibration curve absorbtion.
iii. Calculate the molar absorption value, e, for the complex at 540nm.
iv. Did any of the other metal ions cause any significant variation of absorbance?
v. Calculate the [Cr(VI)] in the water samples by comparison with the calibration curve.
vi. Out of the three methods of reduction, which will be the most environmentally unsound and why?
vii. Besides the methods outlined above, how else could the [Cr(VI)] be calculated?
viii. What will be the shape of the complexed molecule and would the colour be due to d - d orbital shift or electron charge transfer?
ix. What will be the final oxidation state of the Cr(VI) complex and how is this deduced?
x. How were the figures arrived at for the concentrations of the interfering ions (i.e. 0.4484g Co2+). Calculate for all of the ions used (inc. the dichromate).
xi. A full COSHH assessment MUST be given with the written script. Included in this should be reasons why Cr(VI) is so much more toxic than Cr(III).
xii. All bibliographical references should be given.
4 : RESULTS
4.i : The solutions.
All of the solutions were made up as in the revised method sheet before the practical commenced. The DiPC was originally stored in a volumetric flask, but being light sensitive, this had to be disposed of after a few hours. The next DiPC solution was stored in an amber bottle and in a cupboard when not in use.
The solutions were the same for the entire run of practicals to ensure reproducibility of results (except for the DiPC used. This was fresh each time. Any variation would be shown in the calibration curves.)
4.ii : Calibration curve from the standards.
As this practical has been carried out over a number of practical sessions, there are 3 calibration curves. These will be denoted as (i), (ii) and (iii).
(i) This was really just a test run to ensure that the practical was fine, the instrumentation was working and that the basic theory obeyed the Beer law.
(ii) The main one. Using this calibration curve, the interfering ions were assessed and the water samples tested.
(iii) This was performed at LJMU using a bottled water as the test subject. It was carried out on a different machine to the above.
The graphs for (i) and (ii) are absorbance vs. concn (in ppm).
4.iii : The water samples - collection points
The water samples were collected from five parts of Haydock, St. Helens, one from the mains water tap, one from the water still at the University of Salford, LJMU tap water and deionised water and finally a bottled water (commercially available).
The four types were : (1) Rain water pond.
(2) Fishing lake.
(3) Sewerage outlet.
(4) Brook.
4.iv : Preparing the water samples
This was carried out as per revised method. After filtration, a 10cm3 aliquot was placed into a test tube and treated with the DiPC. There was no need for acidification as the pH would be low enough from the addition of conc. sulphuric acid.
4.v : Results for calibration curve with Cr(VI)-DiPC & acid.
The following three tables refer to the absorbance values recorded on the three different occasions when a calibration curve was constructed.
All graphs for the calibration curves and least squares analysis follow the results of the water samples.
| Conc | Abs |
| 0 | 0 |
| 0.400000 | 0.029800 |
| 1 | 0.073350 |
| 2 | 0.172960 |
| 4 | 0.360260 |
| 10 | 0.950740 |
Table 4a. Calibration curve data, week 1
| Conc | Abs |
| 0 | 0 |
| 0.400000 | 0.041390 |
| 1 | 0.097890 |
| 2 | 0.191600 |
| 4 | 0.397510 |
| 10 | 0.951790 |
Table 4b. Calibration curve data. week 2
| Conc | Abs |
| 0 | 0 |
| 0.400000 | 0.031000 |
| 1 | 0.094000 |
| 2 | 0.177000 |
| 4 | 0.372000 |
| 10 | 0.937000 |
Table 4c. Calibration curve data, week 3. (reduction in accuracy)
4.vi : Results from ion interferences.
These results prove the value of the DiPC for determination of Cr(VI).They can all be discounted from the tests at the concentrations used (1.27ppm per ion)
| Metal ion | Absorbance |
| Co2+ | -0.08858 |
| Hg2+ | -0.06404 |
| Fe2+ | -0.09653 |
| Fe3+ | -0.09653 |
| Ba2+ | -0.07724 |
| Cr3+ | -0.07063 |
Table 5. Interference from other ions.
4.vii : Results from water samples
Samples 1-4 are the pond waters, 5 is from University of Salford Physical Chemistry teaching lab, 6 is for the bottled water, 7 and 8 are tap water & deionised water respectively from LJMU. The final three were performed at LJMU.
| Sample | Absorbance | Location | Conc. ppm |
| 1 | 0.08978 | Rain water pond | 0.095 |
| 2 | 0.05449 | Fishing lake | 0.068 |
| 3 | 0.01073 | Sewerage outlet | 0.021 |
| 4 | 0.21443 | Brook | Unknown |
| 5 | 0.00611 | Phys Chem tap | Too low |
| 6 | 0.004 | Bottled water | 0.5 |
| 7 | 0.152 | Tap, LJMU | Unknown |
| 8 | 0.000 | Deionised water | 0.0 |
Table 6. Water sample absorbance values.
4.viii : Cr(VI) reduction by l-Ascorbic acid
This has two results; result 1 from addition of ascorbic acid before complexation with DiPC, result 2 from addition after complexation. The result clearly shows that for this method to be effective, the Cr(VI) has to be reduced by the ascorbic acid prior to complexation.
| Result number | Absorbance |
| 1 | 0.062 |
| 2 | 0.86105 |
Table 7. Reduction by ascorbic acid.
4.ix : Graphs
A further graph (the original from LJMU instrumentation) is in the appendices. The calibration graphs are on the following pages.
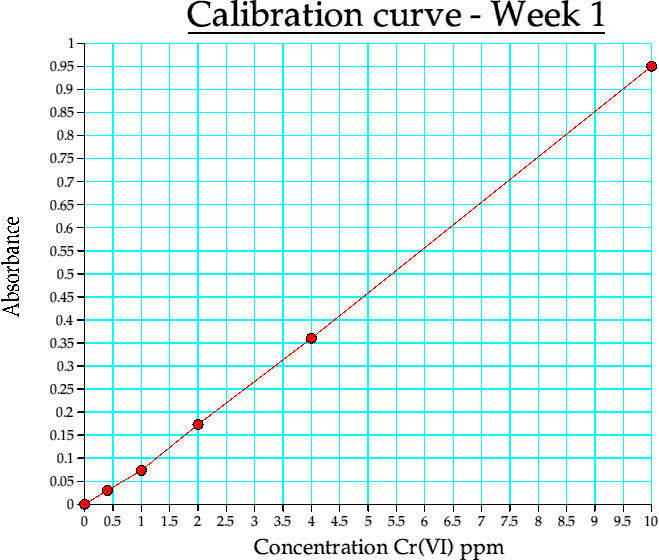
Graph 1. Calibration curve from week 1
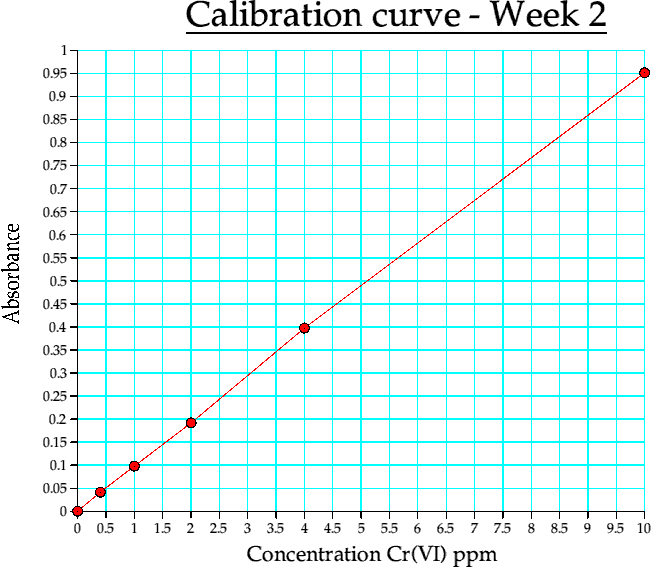
Graph 2. Calibration curve from week 2
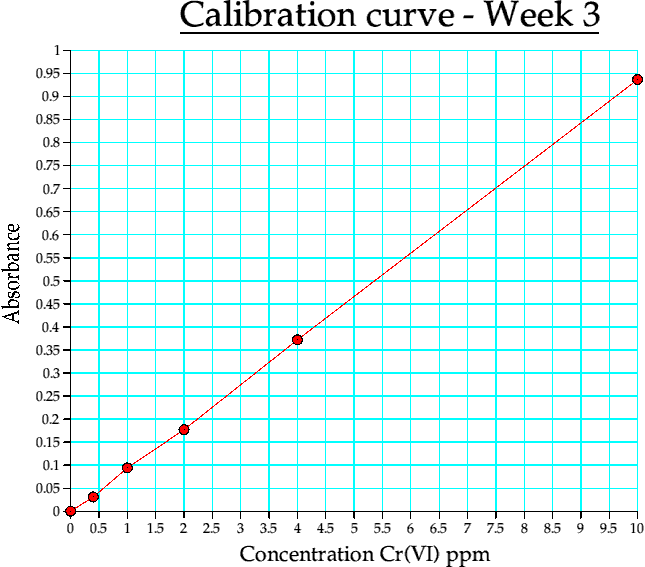
Graph 3. Calibration curve from week 3
4.x: Environmental question
A question is asked in the new method regarding the environmental impact of the three methods and which will have the worst impact. The answer will be a simple one - the Ascorbic acid.
If the Cr(VI) is precipitated out, the solid can be filtered and the waste water run to waste. This may cause some Mg2+ ions to be released. The effect of this will be negligible due to the streams having their own natural buffers, dissolved CO2 in the water and rocks.
Ascorbic acid is completely soluble and any excess will not be compensated using this buffering system in the stream. The ascorbic acid will radically alter the plant growth (l-Ascorbic acid is better known as Vitamin C) as well as an overall increase of water pH.
The addition of Vitamin C supplements to plant and animal life will radically upset the ecosystem of the water stream resulting in a loss of life (quite possibly) from over population, over acidic water and de-oxygenation of the water by increase activity in the stream.
CONCLUSION
The calibration curves on all three occasions give me good reason to say that the experiment is very much reproducable and the quality of the graphs linearity on all occasions that the serial dilution method of obtaining the 1.27ppm is an adequate one.
Analysis of the water samples shows that in all cases, the levels of Cr(VI) are below the allowed amount. The exception was the Brook sample (from Haydock) and LJMU tap water. These will have had a large amount of Iron in. Iron as well as Mercury both interact with the DiPC. This will have masked the overall result of the Cr(VI) reaction.
This practical has been designed (as stated) to be performed by BSc II/HNC II groups as well as the Environmental chemists. As it stands, the practical can be carried out in roughly 2.5 to 3 hours. The questions set at the end should form the basis of a homework or assignment.
It is vital that the chemicals used (especially the DiPC) are fresh and of AR grade. As a side test, I also used the dichromate at SLR and an unspecified quality. Both gave a result for the calibration curve, but towards the top end (the 4 and notably the 10ppm standards) the line tended to become of the true pattern (i.e. not straight). This was not the case with fresh AR grade material. It is not important to the grade of the conc. sulphuric acid. All water MUST only be deionised.
Sources of errors will be in the preparation of the standard solutions by serial dilution and the fact that the DiPC is light sensitive. Any laxness on the part of the student will show in the calibration curve and the least means results.
APPENDIX
iii. Bibliography
1. North West water authority, Water Standards Tables, 1994
2. Wood & Holliday, Inorganic Chemistry - 3rd Ed., pp. 328
3. Belcher & Nutten, Qualitative Inorganic Chemistry - 2nd Ed.
4. Vogel, A. Qualitative Inorganic Chemistry - 3rd Ed.
5. Literature cited value (see original methodology)
6. H.S.E. Chromium, 1983
7. Russian Medical Journal, Russian Health, 1991, pp.32, 61
8. B.M.A. Toxic effects of transition elements on the internal organs. 1961
9. Adapted from Hermann, M.S. J. Chem Ed. 71, 1994, pp. 323-324
10. Lunn G, Samsone, E.B., J. Chem. Ed. 66, 1989, pp. 443
iv : CHROMIUM DATA SHEET
| ELEMENT NAME | Chromium | ORIGIN | Chroma (Greek - colour) |
| ELEMENT TYPE | 1st row Transition metal | STRUCTURE | Cubic body centred |
| DISCOVERED BY | Louis Vauquelin | LOCATION | France |
| YEAR DISCOVERED | 1797 | ||
| ATOMIC NUMBER | 24 | ATOMIC WEIGHT | 51.996 |
| SHELLS | 2,8,13,1 | FILLING ORBITAL | 3d5 |
| MELTING POINT | 1857 °C | BOILING POINT | 2672 °C |
| COVALENT RADIUS | 1.18Å | ATOMIC RADIUS | 1.85Å |
| ATOMIC VOLUME | 7.23cm3 | ||
| ELECTRONEGATIVITY | 1.66 | OXIDATION STATES | 6, 3, 2 |
| 1st IONISATION | 6.766V | 2nd IONISATION | 16.50V |
| 3rd IONISATION | 30.96V | ||
| DENSITY (@ 293K) | 7.19g cm-3 | SPECIFIC HEAT | 0.45 J g-1 K |
| HEAT VAPORISATION | 344.30 kJ mol-1 | HEAT OF FUSION | 16.90 kJ mol-1 |
| ELEC CONDUCTIVITY | 0.0774x106 cm-1 W-1 | THERMAL COND | 0.937 W cm-1 K-1 |
SOURCES : Does not occur freely in nature. Chromite [Fe, Mg(CrO4)] is it's most important mineral.
USES : Used to make stainless steel. It gives colour to rubies and emeralds.
ELEMENT NUCLIDES :
| Nuclide | Abundance (%) | Weight | Spin | Half life | Decay modes |
| 49Cr | 0 | 49 | 2.5 | 42.3m | b+ |
| 50Cr | 4.31 | 49.946 | 0 | Stable | |
| 51Cr | 0 | 50.945 | 7 | 27.70 days | g |
| 52Cr | 83.76 | 51.9405 | 0 | Stable | |
| 53Cr | 9.55 | 52.9407 | 1.5 | Stable | |
| 54Cr | 2.38 | 53.9389 | 0 | Stable |
Data taken from "Periodic Data Sheets" - A C3 computer program.
v. : DATA HAZARD SHEETS
All of the following pages have been taken from the BDH Data Hazard Sheets. They have been reproduced by kind permission.
CHEMICALS USED : Sulphuric acid (18, 0.18 and 3 mol dm-3), l-Ascorbic acid, Potassium chromate (III), Potassium chromate (VI), Propanone, 1,5 Diphenylcarbazide (DiPC), Mercury (II) chloride, Cobalt (II) sulphate, Iron (III) chloride, Ammonium iron (II) sulphate, Barium (II) chloride.
ASSOCIATED RISKS : The higher concentration sulphuric acid (3 and 18mol dm-3) will both cause damage to the skin if in contact. Should the acid be splashed on the skin, it must be wiped off with a dry towel first followed by washing with running water for five minutes. In extreme circumstances, medical assistance should be sought.
SULPHURIC ACID If the acid (any concentration) is splashed into the eyes, the eye must be washed in running water for at least 10 minutes and medical attention sought IMMEDIATELY.
All spillages should first be neutralised with sodium carbonate powder and then wiped up with a damp cloth.
PROPANONE Propanone is highly flammable and should be kept from all sources of ignition (including static). The liquid is volatile and the resulting vapour is a mild anaesthetic. Prolonged exposure may result in giddiness, nausea and collapse (extreme!). The liquid must also not come into contact with the skin as it is a de-greasing agent and may cause sensitisation to occur. Should this happen, the skin affected must be cleaned with soap and water. All solutions must only be used in a fume cupboard.
Disposal of solutions containing propanone should be in the correct waste organic solvents bottle and NOT down the sink.
METAL IONS All of the metal ion solutions are in concentrations of 1.27mg dm-3. This will mean that they are all well within the limits set down (OES maximum TWA exposures). With the exception of the Cr(VI) and Hg(II) solutions, the metal ions at the concentration given above, may be disposed of down the sink without any due cause for alarm. The Cr(VI) and Hg(II) should be greatly diluted when being run to waste.
Due to the low concentrations, any skin contact should just be wiped off with a damp cloth.
1,5 DiPC This substance is light sensitive and is liable to deteriorate if kept in the light. It is only slightly soluble in non-polar solvents and in this practical, it is in a propanone solution.
The solid poses very little hazard to health, the major hazard comes from the carrier solvent (details on other side).
ASCORBIC ACID This poses very little risk in the concentration used for this experiment. All spills should be cleaned with water and run to waste.
OTHER RISKS : The only other significant risk comes from the water solutions used. These have (in four cases) been collected from natural ponds, streams or sewer outlets. As these have not been treated in any way, they must all be treated the same for this assessment.
I have assumed that they will all contain waste organic material and quite a number of undesirable 'bugs'. In addition to this, they may contain trace metal elements (such as iron, mercury, bismuth, chromium (III) and (VI) etc.). It is not my intention to determine if any of these are in the water (except Cr(VI)). The waters will all be filtered and treated with conc. Sulphuric acid (see other side for statement on acid) to dispose of any organic matter in the water. These will have posed the larger risk to health.
The metal ions should now not pose a problem (being in such low concentrations).
Cr(VI)/DiPC complex This complex will only cause a problem as the Diphenylcarbazide (DiPC) is in propanone. At the concentrations used, the complex will contain at the very most 1.27ppm of Cr(VI). The risk from the deterioration of Cr(VI) to the reduction product of Cr(V) will, at this concentration, not give any cause for concern (the maximum limit is far above this value).
All solutions used should be disposed of in a waste organic solvents bottle.
GENERAL Gloves, eye shields and a lab coat must be used when handling any of the materials listed above.
vii : Thanks
My thanks go to the following people for their help with this project in any small way. On the whole, it is academics at both LJMU and University of Salford, but a great thank you must go to my wife, Bev, for proof reading this to ensure that no mistakes have been looked over by the spell checker and no grammatical errors have occurred. She also helped in obtaining the samples.
The list.....
AT LJMU
Dr. Barry Nicholls and Dr. Robert Edwards
AT UNIVERSITY OF SALFORD
Dr. Niel Boag, Dr. Peter Baugh, Dr. S. Simpson, Dr. Tom McC Patterson, Mr. Mike Scanlon and Dr. Brian Iddon.
AT KNOWSLEY COMMUNITY COLLEGE
Dr. D. Roscoe and Mrs. P. Griffiths (for the use of the computer, even after leaving & the DataHazard sheets).
viii : Technology Used
This document was entirely written on an Acorn BBC A3000 using Impression Style (the document processor), !Draw+ (structure of DiPC, adjusting the graphs, basically to smarten them up), !Schema (spreadsheet) and !GraphPro (graph producing package - the spreadsheet is a bit useless at this!).
At University of Salford, the computer used was a Hewlett Packard 286 connected to a H.P. Diode Array Spectroscope (HP 8542A) and HP DeskPlotter. It was set at a static reading of 540nm with a one second scan length. Software used : HP 8953/A UV/VIS O.S. Rev. A.03.00
At LJMU, the spectroscope was a Pye Unicam SP4-800. This manual spectroscope was set to scan between 800 and 290nm. The scan speed was 2nm sec-1 with the graph speed 10secs cm-1. The peak values were obtained at 540nm.
The HTML and subsequent versions have been created using TechWriterPro+ (6.05) on an Acorn RiscPC700.